The FDA doesn't stop monitoring generic drugs after approval-it uses real-time data, manufacturing inspections, and millions of patient reports to catch hidden safety issues. Here's how it works.
Archive: 2026/01
Learn how to read FDA safety alerts without panic. Understand the difference between potential signals and confirmed risks, and how to weigh a drug's benefits against its side effects for smarter health decisions.
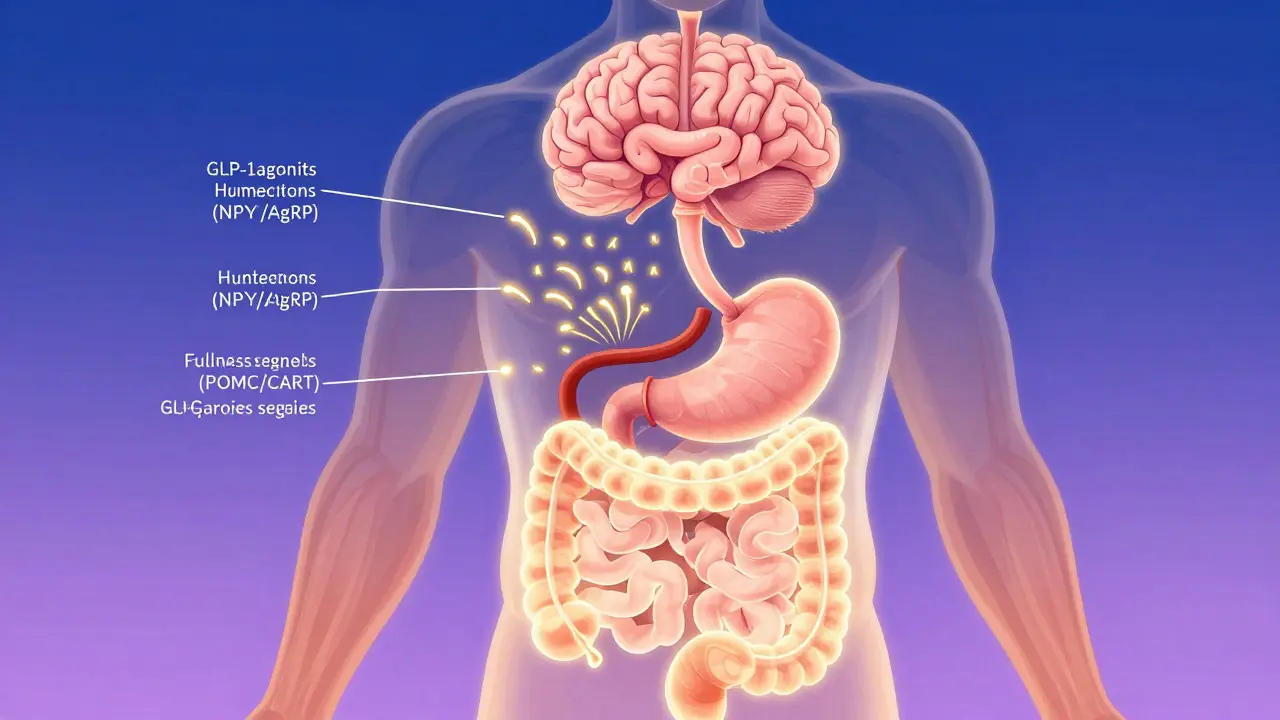
GLP-1 receptor agonists like Ozempic and Wegovy help lower A1C and promote significant weight loss by reducing appetite and slowing digestion. Learn how they work, how they compare to other drugs, and what to expect when using them.
TNF inhibitors like adalimumab and etanercept help control autoimmune diseases but raise concerns about cancer risk. Real-world data shows no overall increase in cancer, but skin cancer risk is slightly higher-especially with adalimumab. Regular skin checks and steroid reduction are key.
Many people misunderstand prescription labels, leading to dangerous dosing errors. Learn the most common mistakes, why they happen, and how to avoid them with simple, practical steps.
EHR integration connects pharmacies and providers to share patient data in real time, cutting medication errors, reducing hospital readmissions, and saving lives. Learn how it works, why most pharmacies still lack it, and what’s changing in 2025.
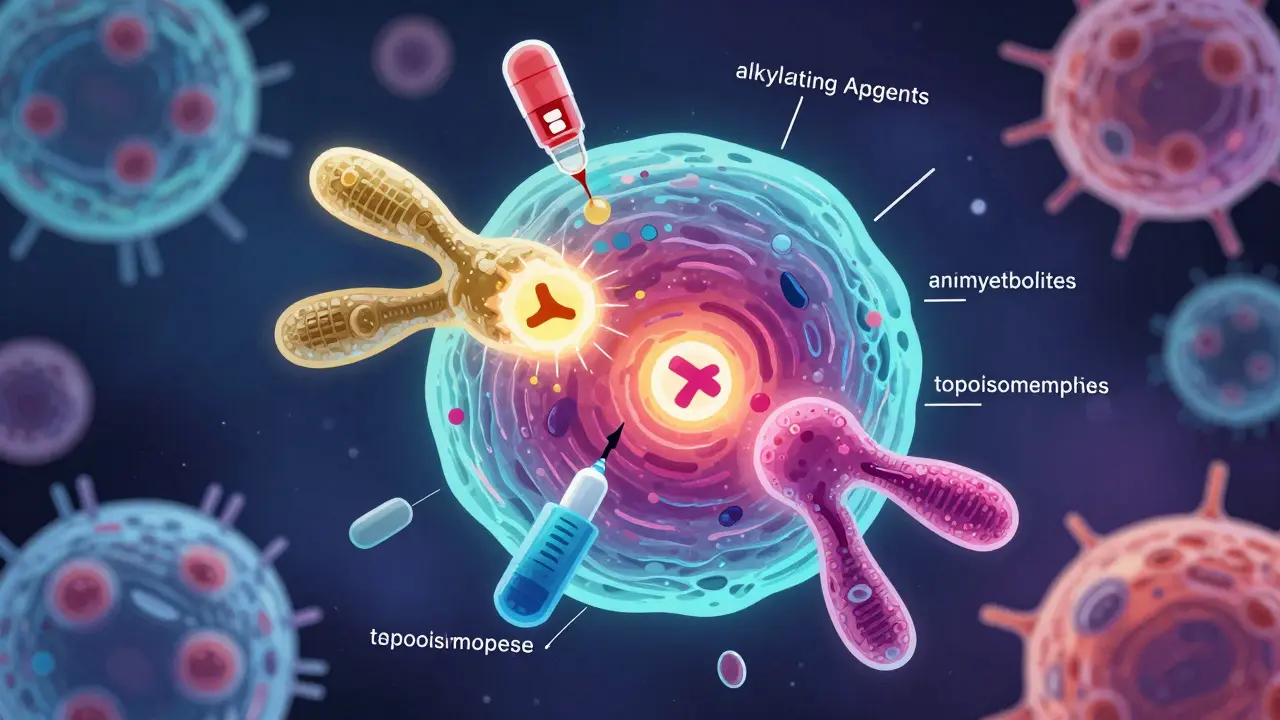
Chemotherapy uses cytotoxic drugs to kill rapidly dividing cancer cells, but also affects healthy cells, causing side effects like fatigue, hair loss, and nerve damage. Learn how it works, why it's still vital in 2026, and how modern care helps manage its impact.
Recognize the early warning signs of serotonin syndrome from antidepressant overdose-tremors, sweating, confusion, and muscle spasms. Act fast to prevent life-threatening complications.
UK NHS substitution laws now require pharmacists to use generic medicines unless blocked by the doctor. New 2025 reforms are shifting care from hospitals to homes - raising safety concerns and access issues for vulnerable patients.
Learn how to access the FDA's FAERS adverse event database for free. Discover tools, limitations, and how to interpret drug safety data without being misled by incomplete reports.