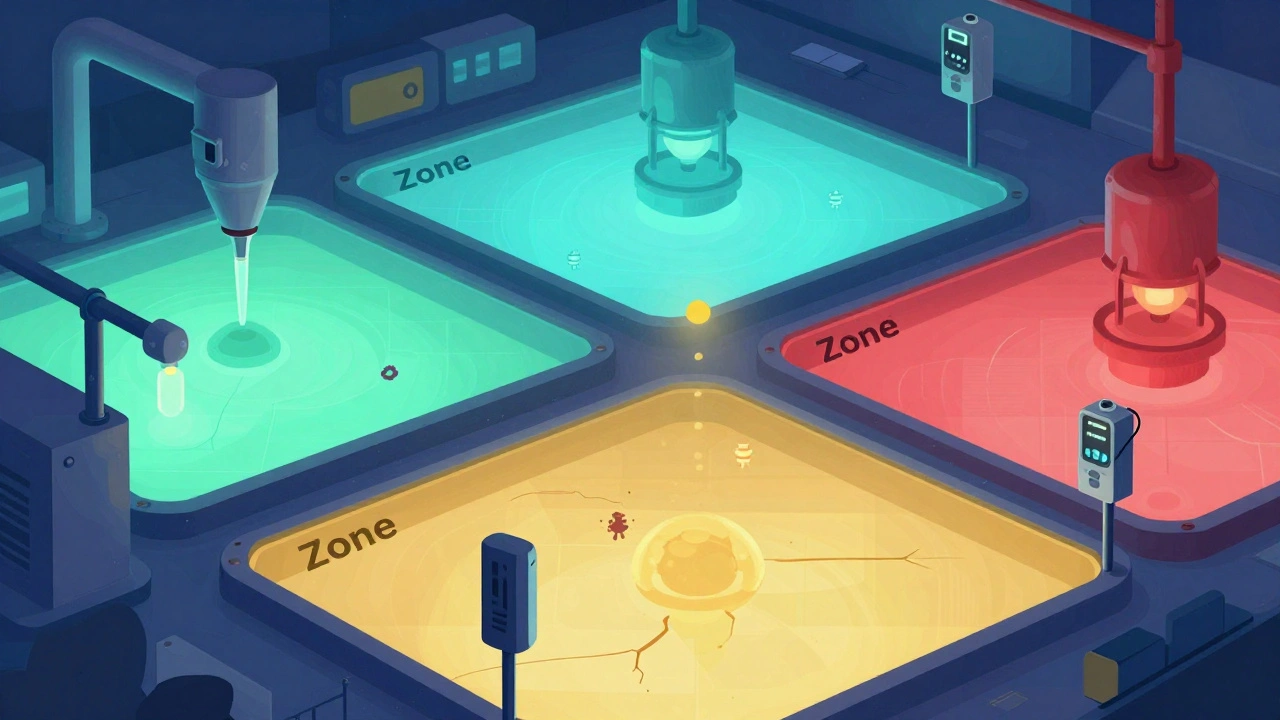
Environmental monitoring is a critical process in manufacturing to detect contamination in air, surfaces, and water before it affects products. Learn how zone-based sampling, industry standards, and new technologies help prevent recalls and ensure safety.
Manufacturing Quality: What Makes a Generic Drug Safe and Reliable
When you pick up a generic pill, you’re trusting that the manufacturing quality, the set of standards and processes that ensure a drug is consistently produced to meet safety and effectiveness requirements. Also known as pharmaceutical production integrity, it’s what keeps your medicine from being weak, contaminated, or fake. It’s not just about what’s inside the pill—it’s about how it was made, where, and under what conditions. The FDA doesn’t just approve the formula; it inspects the factory. A drug made in a dirty facility, with uncalibrated machines, or by untrained workers can fail—even if the active ingredient is perfect.
Manufacturing quality ties directly to generic drugs, medications that are chemically identical to brand-name versions but sold at lower prices. Many people think generics are cheaper because they’re lower quality. That’s not true. The FDA requires them to meet the same FDA standards, rigorous rules governing drug safety, potency, purity, and consistency. Bioequivalence isn’t just a buzzword—it means your generic sildenafil works the same as Viagra, down to how fast it dissolves and how your body absorbs it. But those standards mean nothing if the factory cuts corners. That’s why counterfeit pills keep showing up: they bypass manufacturing quality entirely, slipping through online sellers or grey-market distributors with no oversight.
Manufacturing quality also connects to the drug supply chain, the entire path a medication takes from raw ingredients to your medicine cabinet. A single weak link—like a poorly regulated overseas lab or an unverified shipping partner—can introduce fake or degraded drugs. That’s why the FDA tracks NDC codes, audits foreign plants, and pushes for U.S.-based production. When you hear about a drug shortage, it’s often not because there’s no demand—it’s because the manufacturer failed an inspection or couldn’t meet quality controls.
What you’ll find in these posts isn’t just theory. It’s real stories: how a generic drug passed FDA testing but still looked different because of trademark laws, how a patient spotted a fake pill online and reported it, how a shortage forced pharmacies to switch brands—and why that switch was safe because of manufacturing quality. You’ll learn how to spot red flags in your meds, why your insurance pushes for generics, and how to check if your drug is being made under proper conditions. This isn’t about trusting labels. It’s about understanding what keeps those labels trustworthy.
Environmental monitoring in manufacturing prevents contamination by testing air, surfaces, and water for microbes, chemicals, and particles. Learn how zone systems, sampling methods, and regulations ensure product safety in pharma, food, and cosmetics.