Environmental Monitoring in Manufacturing: Testing Facilities for Contamination
When you buy a bottle of medicine, a ready-to-eat meal, or a jar of face cream, you assume it’s safe. But behind that assumption is a hidden system of checks-environmental monitoring-that stops contamination before it ever touches the product. This isn’t optional. It’s required. And in manufacturing, getting it wrong can mean recalls, lawsuits, or worse-people getting sick.
Why Environmental Monitoring Matters
Environmental monitoring isn’t about checking if the floor is clean. It’s about proving that the entire environment around your product can’t contaminate it. The CDC says it clearly: monitoring surfaces, air, and water helps confirm the presence of hazards and proves you’ve stopped them. In food plants, a single Listeria monocytogenes particle on a conveyor belt can trigger a nationwide recall. In pharmaceutical labs, a single fungal spore in the air can ruin an entire batch of injectable drugs. The FDA and EMA don’t just recommend this-they enforce it. The U.S. Food Safety Modernization Act (FSMA) and EU GMP Annex 1 require continuous monitoring. Companies that skip it risk shutdowns. In 2022, the USDA estimated foodborne illnesses tied to poor environmental control cost the U.S. economy $77.7 billion. That’s not a number. That’s hospitals, lost jobs, and broken trust.The Zone System: How Contamination Risk Is Ranked
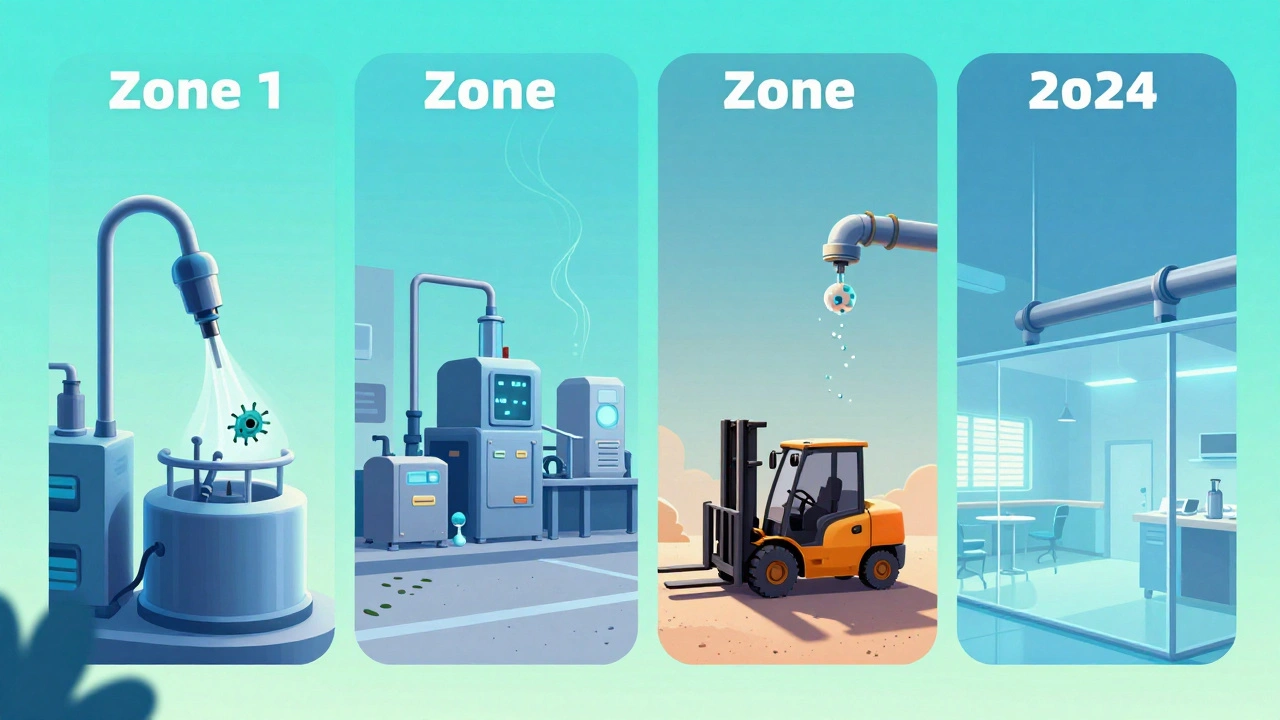
Every serious facility uses a zone system to prioritize where to test. It’s simple, but it saves lives.- Zone 1: Direct food or drug contact surfaces-slicers, mixers, filling nozzles, packaging tools. These are high-risk. If something grows here, it goes straight into the product.
- Zone 2: Surfaces near Zone 1-equipment housings, refrigeration units, nearby carts. Contamination here can drift or splash into Zone 1.
- Zone 3: Remote but still in the production area-forklifts, overhead pipes, walls. Surprising? A 2013 study by PPD Laboratories found that 62% of all contamination events traced back to Zone 3 floors.
- Zone 4: Outside the production area-break rooms, hallways, offices. Low risk, but still monitored to catch cross-contamination.
What You’re Testing For
Different industries test for different things, but the core targets are the same:- Microorganisms: Bacteria like Listeria, Salmonella, and E. coli. Yeasts and molds are common in cosmetics and food.
- Particulates: Dust, fibers, skin flakes. Critical in sterile drug manufacturing. The FDA requires continuous air monitoring in ISO Class 5 cleanrooms.
- Chemicals: Residues from cleaners, lubricants, or packaging materials. Tested with GC, HPLC, or UPLC systems.
- Metals: Lead, nickel, or aluminum leaching from equipment. Detected using Inductively Coupled Plasma (ICP) analyzers.
- Water quality: Purified water in pharma must meet USP <645> standards. Conductivity and total organic carbon (TOC) are checked daily.

How Sampling Works
Sampling sounds simple-wipe a surface, collect air, send it to a lab. But it’s easy to mess up.- Swabs must be sterile. If the swab itself is contaminated, you get a false positive.
- Air samplers use liquid impingers or solid impactors. They pull hundreds of liters of air in minutes. The results? Organisms per cubic meter (CFU/m³).
- For Zone 1, samples are taken daily. Zone 2? Weekly. Zone 3 and 4? Monthly. But frequency depends on risk-not a calendar.
Industry Differences: Pharma vs. Food vs. Cosmetics
Not all facilities are built the same.- Pharmaceutical: Must follow EU GMP Annex 1 (updated August 2023). Requires real-time monitoring of temperature, humidity, and particles. Continuous air sampling in cleanrooms. Water systems are tested hourly. The goal? Zero viable organisms in sterile products.
- Food Processing: Must follow USDA’s Listeria Rule (9 CFR part 430). Focus is on RTE (ready-to-eat) foods. Zone 1 testing for Listeria is mandatory at least weekly. FDA inspectors show up with swabs and check for Salmonella in drains.
- Cosmetics: Less strict than pharma, but still regulated. Focus on mold, yeast, and bacterial contamination in creams and lotions. Water-based products are high risk. Many use the same zone system as food plants.
Biggest Mistakes Facilities Make
Even smart companies fail. Here’s what goes wrong:- Sampling technique: 68% of facilities don’t train staff properly. Swabs aren’t sterilized. Air samplers aren’t calibrated. Results are useless.
- Data silos: ATP results, microbial data, allergen tests, and chemical reports live in different spreadsheets. No one connects the dots. That’s why 37% of facilities can’t spot patterns.
- Over-monitoring: Some think more tests = better safety. But PPD Labs found that a focused, fit-for-purpose program works better than blanket testing. Less is often more.
- Ignoring Zone 3: Floors, drains, and pipes get ignored. Yet they’re the #1 source of contamination events.
What’s Changing in 2025
The rules are getting tighter.- Real-time data: EU Annex 1 now requires continuous monitoring with automated trending. Manual logs are outdated.
- AI and machine learning: Systems now analyze months of data to predict contamination risks before they happen. By 2027, 38% of monitoring systems will use AI-up from 12% in 2022.
- Next-generation sequencing: Instead of waiting days for culture results, labs can now identify pathogens in under 24 hours using DNA sequencing. The FDA is pushing for this in high-risk facilities.
- Antibiotic resistance: 19% of Listeria strains from food environments now resist multiple antibiotics. Monitoring now includes resistance profiling.
How to Start or Improve Your Program
If you’re building a program from scratch-or fixing a broken one-here’s how:- Map your zones. Be specific. Draw floor plans. Label every surface.
- Identify your top 3 risks. Is it Listeria? Mold? Metal leaching? Focus there first.
- Train your team. At least 40 hours of hands-on sampling training. No exceptions.
- Use ATP for quick sanitation checks-but always confirm with cultures.
- Integrate your data. One dashboard. One system. No spreadsheets.
- Review results weekly. Don’t wait for an audit. If something spikes, investigate immediately.
- Don’t forget Zone 3. Clean your floors. Unclog your drains. Those are your hidden threats.
What Success Looks Like
In 2013, PPD Labs tracked over 10,000 environmental samples across U.S. and Irish facilities. Only seven events-less than 0.01%-exceeded action limits. Why? They didn’t test everything. They tested smart. They trained well. They fixed problems fast. That’s the goal. Not perfection. Not endless testing. Just control. If your facility can say, "We know what’s in our air, on our surfaces, and in our water-and we’ve stopped it before it touched the product," then you’re not just compliant. You’re safe.What’s the difference between environmental monitoring and product testing?
Environmental monitoring checks the environment around the product-surfaces, air, water-to prevent contamination before it happens. Product testing checks the final item after production. Both are needed, but environmental monitoring stops problems at the source. You can’t test contamination out of a product-you have to stop it from getting in.
How often should I test Zone 1 surfaces?
Zone 1 surfaces should be tested daily or at least every production shift, especially in food and pharmaceutical facilities. The FDA requires weekly testing for Listeria in RTE food Zone 1 areas, but many facilities test daily as a best practice. If your process runs 24/7, daily testing is non-negotiable.
Can ATP testing replace microbial testing?
No. ATP testing detects organic material quickly, which helps verify cleaning effectiveness. But it doesn’t tell you if harmful bacteria like Listeria or Salmonella are present. Microbial testing is still required for regulatory compliance and to identify specific pathogens. Use ATP for speed, cultures for accuracy.
Why are Zone 3 and 4 surfaces important if they don’t touch the product?
Contamination doesn’t always move in straight lines. Dust from a floor can get stirred up by a forklift and land on a machine. Water from a leaky pipe can drip onto a cart that touches a product. A 2013 study found 62% of contamination events originated in Zone 3. Ignoring these areas is like locking your front door but leaving your windows open.
What’s the biggest cost of poor environmental monitoring?
It’s not the cost of tests or equipment. It’s the cost of a recall. A single Listeria outbreak in food can cost over $10 million in lost sales, legal fees, and brand damage. In pharma, a contaminated batch can mean shutting down a production line for weeks. The CDC says 87% of environmental-linked foodborne outbreaks could’ve been prevented with proper monitoring. That’s not a risk-it’s a liability.
Do small manufacturers need a full environmental monitoring program?
Yes-if they’re regulated. Even small food or cosmetic makers must comply with FDA or USDA rules. The good news? You don’t need a full lab. Start with Zone 1 and 2. Use third-party labs for testing. Train one person to handle sampling. Focus on your biggest risk. A simple, well-executed program beats a half-done complex one.
What’s the future of environmental monitoring?
The future is real-time, predictive, and automated. AI will analyze historical data to flag risk zones before contamination happens. DNA sequencing will identify pathogens in hours, not days. Sensors will monitor air quality and humidity continuously. Facilities that adopt this early will avoid recalls, reduce waste, and build trust faster than competitors still using paper logs.

Ada Maklagina
Zone 3 being the silent killer is wild. I used to work in a food plant and no one cleaned the floor drains until an audit flagged it. Then boom-recall. Just saying, dirt doesn’t care how big your budget is.
Harry Nguyen
So we’re spending billions to make sure your yogurt doesn’t have a single bacteria while China dumps industrial waste into rivers and no one says a word? This is the cost of overregulation. We’re turning factories into sterile labs while the real threats go ignored.
Katie Allan
What’s beautiful here is how simplicity wins. You don’t need fancy tech to save lives-you need clear zones, trained people, and the humility to admit that a dirty floor can kill. This isn’t science fiction. It’s basic human care.
Deborah Jacobs
I swear, the moment I saw that 62% of contamination came from Zone 3, I remembered my uncle’s tiny taco shop. He’d scrub the grill daily but let the grease trap fester for months. One summer, three people got sick. He didn’t even know why. That stat? It’s not data-it’s a scream. Clean the damn floor. Train the damn staff. Stop pretending the edges don’t matter.
James Moore
Let’s be perfectly clear: this entire system is a bureaucratic overreach disguised as public safety. The FDA, the EMA, the USDA-they’re not protecting you; they’re protecting their budgets. ATP testing? It’s a band-aid. DNA sequencing? A costly toy. Real safety comes from common sense, not compliance checklists. And yet, we’re told to bow to these institutions like they’re infallible. What happened to personal responsibility? What happened to trusting the worker on the line? We’ve outsourced vigilance to spreadsheets and sensors, and now we’re surprised when things go wrong.
Kylee Gregory
I like how this breaks it down without jargon. Zone 3 being the hidden villain is such a good reminder-sometimes the most dangerous things aren’t the ones you see, but the ones you ignore because they’re ‘out of the way.’
Lucy Kavanagh
You know who really controls this? The lab companies. They sell the swabs, the machines, the tests. If you stopped all monitoring tomorrow, their stock would crash. Coincidence? I don’t think so. They want you scared. They want you buying new gear every year. Real safety? It’s soap, water, and discipline. Not a $50,000 sensor.
Chris Brown
The notion that environmental monitoring is ‘non-negotiable’ is precisely the kind of authoritarian thinking that erodes industrial autonomy. We have become a society that equates compliance with virtue. But virtue cannot be legislated through swabs and air samplers. True integrity lies in character-not in audit trails.
Stephanie Fiero
If you’re a small biz owner reading this-don’t panic. You don’t need a PhD to do this right. Start with Zone 1. One person trained. One checklist. One daily wipe-down. That’s 80% of the battle. I’ve seen it. You got this.
ashlie perry
They’re hiding the truth. The real reason they monitor so hard? So they can blame YOU when something goes wrong. The same companies that make the equipment also own the labs. They profit from fear. You think your face cream is safe? Think again.
Juliet Morgan
I work in a small cosmetic lab and this post made me cry. Not because I’m overwhelmed-because I finally feel seen. We’ve been doing this with duct tape and hope for years. Thanks for saying what we’ve been too tired to say out loud.
Norene Fulwiler
In my country, we don’t have fancy sensors. We have grandmas who wipe surfaces with cloth and vinegar before sunrise. They don’t know what ATP is. But they know when something feels wrong. Maybe the real lesson here is: trust the human who shows up every day.
William Chin
It is imperative to underscore that the aforementioned regulatory frameworks, namely EU GMP Annex 1 and FSMA, are not merely advisory in nature; they constitute legally binding mandates under the purview of international trade and public health law. Noncompliance, therefore, constitutes a material breach of fiduciary duty to the consuming public.