Environmental Monitoring: Testing Facilities for Contamination in Manufacturing
When you buy a bottle of medicine, a ready-to-eat meal, or a tube of lotion, you expect it to be safe. But behind every product is a factory - a place where air, water, surfaces, and equipment can quietly harbor harmful microbes or chemicals. Environmental monitoring is how manufacturers catch those threats before they reach you. It’s not optional. It’s the line between a recall and a safe product.
What Environmental Monitoring Actually Does
Environmental monitoring isn’t just swabbing surfaces and hoping for the best. It’s a structured system that checks for contamination in air, water, and surfaces across every part of a production facility. The goal? Find problems early - before they ruin a batch, trigger a recall, or worse, make someone sick. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) treat this like a critical control point. In pharmaceuticals, food, and cosmetics, it’s legally required. The CDC calls it one of the most effective ways to prevent foodborne illness outbreaks, which cost the U.S. over $77 billion a year. That’s not a small number. That’s a system failure on a national scale. Monitoring doesn’t just look for bacteria. It tracks airborne particles, metal residues, chemical traces, and even water purity. Different tools do different jobs:- Swabs and sponges collect microbes from surfaces
- Air samplers pull in liters of air to count fungal spores or bacteria per cubic meter
- Total organic carbon (TOC) meters check water purity in pharmaceutical lines
- ICP machines detect heavy metals like lead or mercury
- ATP tests give results in seconds - showing if a surface is clean enough to start production
The Zone System: Risk-Based Monitoring
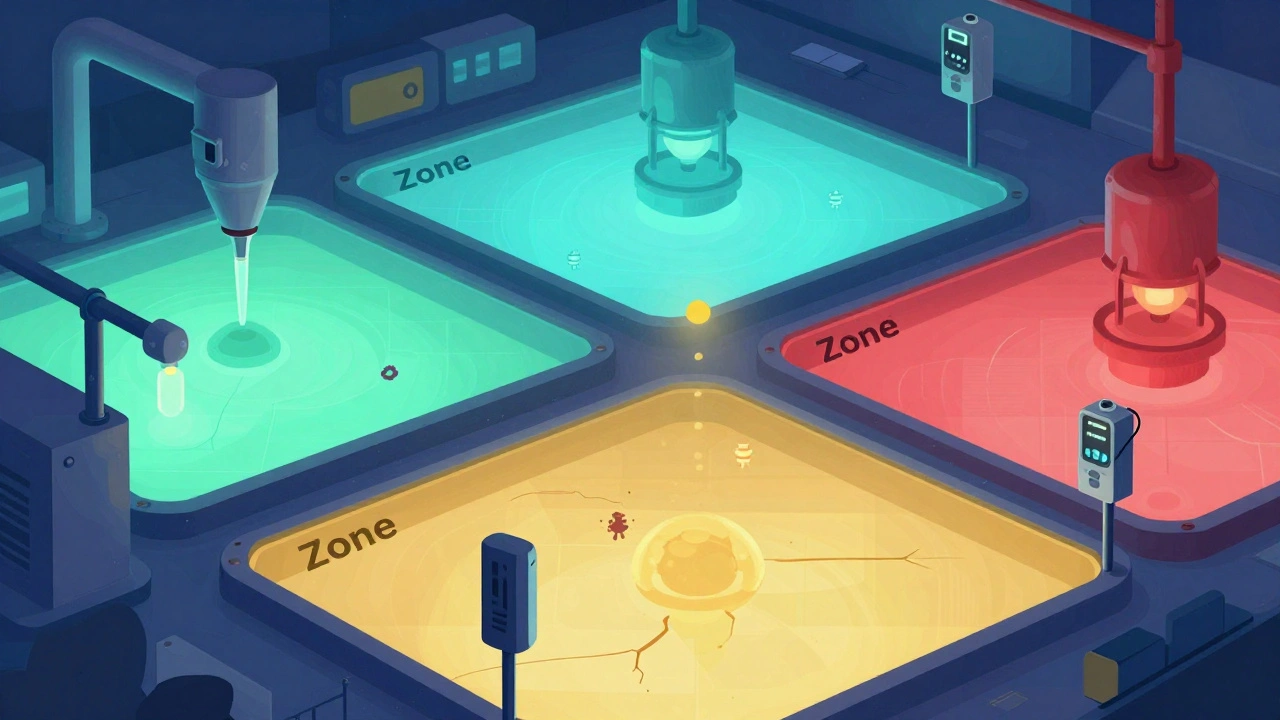
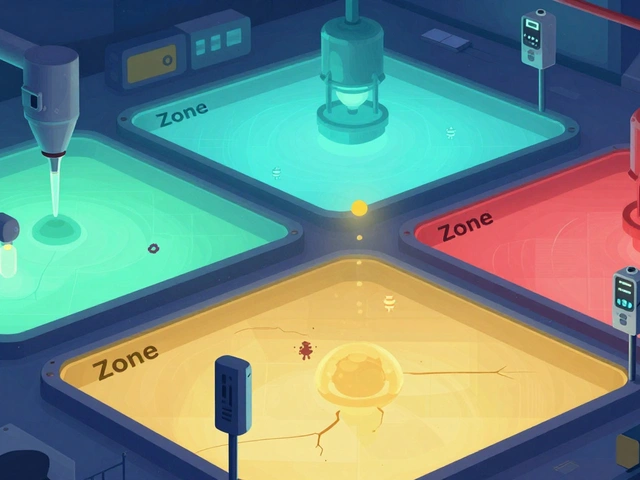
Not all surfaces are created equal. That’s why every serious facility uses the zone classification system. Think of it like a security level for contamination risk.- Zone 1: Direct food or drug contact surfaces - slicers, mixers, filling nozzles. These are sampled daily or every shift. A single pathogen here can contaminate thousands of units.
- Zone 2: Non-contact surfaces near Zone 1 - equipment housings, refrigeration units, conveyor belts. Tested weekly. This is where most cross-contamination starts.
- Zone 3: Remote but still inside production areas - forklifts, storage racks, walls near the line. Tested monthly. Surprisingly, PPD Labs found that floors (a Zone 3 surface) caused 62% of all contamination alerts in bioassay labs.
- Zone 4: Outside production - restrooms, break rooms, hallways. Tested quarterly. Low risk, but still monitored because people move between zones.
Industry Differences: Pharma vs. Food vs. Cosmetics
Not all industries monitor the same way. The rules change based on what’s being made. In pharmaceuticals, cleanrooms must meet ISO Class 5 standards - meaning fewer than 3,520 airborne particles per cubic meter. Continuous particle counters run 24/7. Temperature and humidity are logged every minute. The EU’s Annex 1 (updated August 2023) demands real-time data trending - no more manual logs. Food facilities focus on pathogens. Ready-to-eat foods like deli meats or salads must test weekly for Listeria monocytogenes under USDA’s Listeria Rule. Salmonella is another top target. Air sampling is less common - they care more about what’s touching the food. Cosmetics sit in the middle. They don’t need sterile cleanrooms, but they must prove they’re not harboring mold or yeast. A contaminated eye cream can cause infections. That’s why many cosmetic makers now use the same zone system as food plants. Water systems differ too. Pharma uses purified water tested for TOC and conductivity under USP <645>. Food plants rely on municipal water safety - tested by local utilities, not the factory.
What Goes Wrong in Real Factories
Even with good rules, execution fails. Here’s what breaks down:- Sampling technique: 68% of facilities don’t sterilize samplers properly. A dirty swab gives false negatives.
- Zone confusion: 42% of facilities can’t agree on what’s Zone 1 vs. Zone 2. Training gaps cause this.
- Data silos: ATP results, microbiology reports, and allergen tests live in different spreadsheets. No one connects the dots. That’s a red flag for inspectors.
- Understaffing: Medium-sized food plants spend $15,000-$25,000 a year on testing and need 2-3 full-time staff. Many small plants can’t afford that.
Technology Is Changing the Game
The future of environmental monitoring isn’t just more tests - it’s smarter data. Next-generation sequencing (NGS) can now identify microbes from a swab in under 24 hours instead of 3-4 days. That’s huge. Instead of guessing what’s growing in a petri dish, you get a full DNA profile. Is it Listeria? Is it resistant to antibiotics? You’ll know fast. AI is stepping in too. Systems now analyze years of monitoring data to predict where contamination is likely to occur. If a floor swab always spikes in March, the system flags it before it happens. That’s predictive control - not just reactive. The EMA now requires real-time data logging in pharmaceutical plants. No more paper logs. Sensors stream temperature, humidity, and particle counts directly to cloud dashboards. If a filter fails at 2 a.m., the system alerts the team before morning production starts. By 2027, nearly 40% of monitoring systems will use AI. That’s up from 12% in 2022. The market for this tech is growing at 11.8% a year - and it’s not just for big pharma. Even mid-sized food plants are adopting automated samplers and cloud-based data platforms.How to Build a Solid Program
If you’re setting up environmental monitoring, start here:- Map your zones. Don’t guess. Walk every inch. Mark every surface that could touch product or harbor moisture.
- Define sampling frequency. Zone 1: daily. Zone 2: weekly. Zone 3-4: monthly. Adjust based on history - if a drain always tests positive, test it twice a week.
- Train your team. The FDA recommends 40 hours of hands-on training. Practice swabbing. Learn how to sterilize samplers. Show them how to label samples correctly.
- Use ATP + microbiology together. ATP for quick clearance. Microbiology for confirmation. Don’t pick one.
- Integrate your data. Use software that pulls ATP, swab, and air results into one dashboard. If a swab comes back positive, the system should flag all recent ATP readings in that area.
- Review monthly. Look for trends. Is contamination increasing in one zone? Is a new machine causing spikes? Fix it before the inspector shows up.

Why This Matters Beyond Compliance
Yes, regulators require it. But the real win? Trust. A single contamination event can destroy a brand. In 2022, a food plant in Wisconsin lost $18 million in recalls after Listeria was found on a conveyor belt. They didn’t test Zone 2 often enough. The CDC says 87% of outbreaks like this could’ve been stopped with proper monitoring. It’s not about passing an audit. It’s about knowing your product is safe - every time. That’s what keeps customers coming back. That’s what keeps your factory open.Frequently Asked Questions
What’s the difference between environmental monitoring and product testing?
Product testing checks the final item - like a bottle of medicine or a bag of chips - for contamination. Environmental monitoring checks the factory itself: the air, the floors, the machines. You can have clean products but a dirty environment. That’s why both are needed. Environmental monitoring finds the source so you can fix it before the next batch is made.
How often should I test Zone 1 surfaces?
Zone 1 surfaces should be tested at least daily in high-risk environments like pharmaceutical cleanrooms or ready-to-eat food lines. In lower-risk settings, testing every shift or every other day is common. The key is frequency based on risk - if a surface is touched by bare hands or exposed to moisture, test it more often.
Can ATP testing replace microbial swabs?
No. ATP tests show organic residue - dirt, grease, food bits - which often means microbes are present. But they don’t identify specific pathogens like Listeria or Salmonella. Microbial swabs are still required for regulatory compliance. Use ATP for quick clearance between shifts. Use swabs to confirm safety before releasing product.
Why do floors cause so many contamination events?
Floors are high-traffic areas. People walk on them, carts roll over them, and water drips from equipment above. Even if they’re not direct contact surfaces, they kick up particles and harbor microbes like mold and bacteria. PPD Labs found floors caused 62% of all contamination alerts in labs - even more than equipment. Cleaning them isn’t enough; you need regular sampling and proper drainage.
What’s the biggest mistake facilities make with environmental monitoring?
Treating it as a checklist instead of a system. Many facilities collect samples, send them to a lab, and forget about them. The real value comes from analyzing trends, linking data across zones, and using results to improve cleaning schedules and training. If you’re not acting on the data, you’re just paying for paperwork.
Is environmental monitoring worth the cost?
Absolutely. The average cost for a medium-sized facility is $15,000-$25,000 per year. A single product recall can cost millions. One food recall in 2021 cost a company $18 million in lost sales, legal fees, and rebranding. Environmental monitoring is insurance. It’s far cheaper than the alternative.

ian septian
Just test Zone 1 daily. Use ATP for quick checks. Run swabs weekly. Train your team. Log everything. Done.
Mona Schmidt
This is one of the clearest breakdowns of environmental monitoring I’ve ever read. The zone system explanation alone should be mandatory reading for every QA manager. I especially appreciate how you tied floor contamination to real data-62% is staggering. Many facilities still treat floors as ‘background noise,’ but they’re often the silent source of cross-contamination. Training staff to see the whole system, not just their assigned swab spot, is the real game-changer.
Also, the point about data silos? Spot on. I’ve seen labs with ATP results in Excel, microbiology in LIMS, and air sampling in a binder. No integration means no trend analysis. That’s not compliance-it’s theater. Software that links all data streams isn’t a luxury; it’s the minimum standard now.
And kudos for calling out ATP vs. microbial testing. Too many think ATP replaces culture plates. It doesn’t. It’s a flashlight, not a microscope. You need both. One tells you if it’s clean enough to start. The other tells you what’s lurking if you skip cleaning.
For anyone reading this and thinking, ‘We’re too small for this’-you’re wrong. Start with one zone. One surface. One swab. Build from there. You don’t need a $50k system to stop a recall. You just need to care enough to track it.
Sarah Gray
Of course you wrote this like it’s gospel. But let’s be real-this whole system is just expensive theater designed to keep consultants employed. Most contamination events come from human error, not faulty swabs. You think a $20,000 annual monitoring budget prevents outbreaks? It just shifts blame from the worker who didn’t wash hands to the ‘Zone 3 floor that wasn’t sanitized enough.’
And don’t get me started on NGS. Next-generation sequencing? In a $12/hour food plant? You’re kidding. This is pharma elitism dressed up as ‘best practice.’ The real solution? Hire better people. Pay them more. Stop pretending tech fixes laziness.
Kathy Haverly
Oh wow. Another ‘compliance is life’ sermon. Let me guess-you’ve never worked a shift where the machine broke down at 3 a.m. and you had to clean it with a rag and prayer because the ATP meter was broken? This whole post reads like a vendor’s sales pitch. ‘Use our software!’ ‘Log everything!’ ‘Real-time dashboards!’
Meanwhile, 70% of small manufacturers are drowning in paperwork because some FDA auditor once said ‘trends’ matter. But when your only employee is also the owner, the janitor, and the QA lead-trends are a fantasy. This isn’t about safety. It’s about control. And control is just another word for fear.
And who decided floors are Zone 3? In my last job, the ceiling dripped. So was it Zone 1? Zone 4? Or just ‘bad luck’? This system is arbitrary. And expensive. And useless when the people running it are overworked and underpaid.
Michael Robinson
It’s funny how we think more data means more safety. But data doesn’t care. It just sits there. What matters is whether someone looks at it, feels something, and changes their behavior. A swab result is just ink on paper until someone says, ‘Wait-that’s the same spot every time.’ Then it becomes a story. And stories make people act.
Maybe the real problem isn’t the monitoring. It’s that we stopped listening to the people who actually clean the floors. They know where the water leaks. They know which door sticks and kicks up dust. They know when the machine hums wrong. But we treat them like background noise. That’s the real contamination.
Arun Kumar Raut
Great breakdown. I work in a small spice facility in India, and we just started Zone mapping last month. We didn’t even realize our spice grinders were Zone 1-thought they were Zone 2. Now we test them daily. We use cheap ATP strips from Alibaba-$0.50 each-and they’ve cut our downtime by half. No fancy software. Just a notebook and a team that actually talks to each other.
Also, floors? We used to clean them once a week. Now we clean them twice a day and swab every Friday. Mold was growing under the conveyor belt. No one noticed until we started sampling. Now we fix leaks before they drip.
It’s not about being perfect. It’s about being consistent. And listening.
Evelyn Pastrana
So let me get this straight… we’re spending $25K a year to test floors so we don’t get sued when someone gets sick from a salad? And this is supposed to be ‘trust’? 🤡 I mean, I get it-safety matters. But this feels like paying for a bulletproof vest… while driving a car with no brakes.
Meanwhile, the real killer? Processed food full of sugar and chemicals. But hey, at least the conveyor belt was swabbed. 😌
Elliot Barrett
Wow. 15 pages of fluff. Where’s the data? Who funded this? Which lab did the ‘62% floor contamination’ study? PPD Labs? Never heard of them. Sounds like a made-up name. And ‘FDA says’? Where’s the citation? Link the regulation. Show me the code.
This reads like a blog post written by a marketing intern who Googled ‘FDA environmental monitoring’ and thought it sounded impressive. No hard numbers. No references. Just vibes.
If this is what passes for ‘deep analysis’ now, no wonder people don’t trust science.
Andrea Petrov
Let me guess-you work for a company that sells those $50,000 air samplers. This whole thing is a sales pitch disguised as education. Real talk: the FDA doesn’t care about your swabs. They care about your profits. They know contamination happens. They just want you to have paperwork when the lawsuits come.
And NGS? Please. DNA sequencing in a food plant? That’s not innovation-it’s surveillance. Who’s watching your data? Who owns it? What if it gets hacked? What if your ‘predictive AI’ flags your own facility as ‘high risk’ and gets you shut down by an algorithm?
This isn’t safety. It’s control. And they’re selling it to you as salvation.
Graham Abbas
Man, this post hit me right in the gut. I used to work in a cosmetic lab in London-we had a leaky pipe above Zone 2. For months, we kept getting yeast positives. We changed the swabs. We cleaned more. We blamed the humidity. Then one day, the janitor-old man with 30 years there-said, ‘The pipe’s been dripping since ’09. You ever check the ceiling tiles?’
We did. Mold growing like a sponge. We replaced it. Problem solved.
That’s the thing no one talks about: the people who clean the place know more than the people who write the protocols. We treat them like ghosts. But they’re the ones holding the line.
This isn’t about tech. It’s about listening.
Nikhil Pattni
Bro, you missed the big picture. Environmental monitoring is just the tip of the iceberg. The real issue is global supply chains. Where do your swabs come from? China? India? Are they sterile? Or are they sitting in a warehouse in Mumbai with 90% humidity for 3 weeks? You think your ATP meter is accurate if the swab itself is contaminated? You’re not monitoring contamination-you’re monitoring a chain of failures.
And don’t get me started on AI. AI learns from data. But if your data is garbage-like a janitor who writes ‘clean’ because he’s tired-then your AI will say ‘everything’s fine.’ That’s not predictive. That’s delusional.
Also, Zone 3? In India, we don’t have Zones. We have ‘where the boss walks.’ If he walks there, it’s clean. If not? It’s fine. That’s the real system.
And why are you using USP? We use ISO 22000. Different rules. Different world. This whole post is American-centric. Global manufacturing doesn’t care about your FDA.
precious amzy
One must question the epistemological foundations of this entire paradigm. Environmental monitoring, as currently practiced, is a performative ritual of quantified control-a neoliberal fetishization of data as a substitute for ethical responsibility. The invocation of ‘safety’ functions discursively to obscure the underlying commodification of human health under late-stage industrial capitalism.
Furthermore, the notion that ATP testing can be ‘paired’ with microbiological analysis reveals a profound ontological confusion: the reduction of microbial life to measurable units is an act of epistemic violence. Life cannot be quantified. Only its absence can be simulated.
And the ‘zone system’? A spatial hierarchy designed to distribute blame while preserving institutional power. The floor is not a ‘Zone 3’-it is a site of colonial subjugation, where the labor of the unseen is rendered invisible through bureaucratic taxonomy.
This is not safety. It is spectacle.
Carina M
While the content is superficially well-structured, it fails to address the fundamental ethical lapse in modern manufacturing: the normalization of risk. The fact that $15,000–$25,000 is considered an ‘acceptable’ cost for preventing recalls reveals a moral bankruptcy. When human health is reduced to a line item in an audit checklist, we have already lost.
Regulatory compliance is not virtue. It is the bare minimum of decency. And yet, this post treats it as an achievement. The real failure is not in the swabs or the zones-it is in the collective surrender to ‘good enough.’
One must ask: if this system were truly effective, why do outbreaks persist? Why are recalls increasing? The answer is not in better technology-but in the abandonment of moral courage.
William Umstattd
Let’s be honest-this whole system is a scam. You know how many times I’ve seen a ‘positive’ swab get ‘retested’ until it came back clean? Or a lab ‘adjusting’ results because the plant was getting audited? This isn’t science. It’s theater.
And don’t get me started on the ‘AI predicts contamination’ nonsense. That’s just fancy guesswork dressed up in jargon. If your AI can predict a mold spike in March, why didn’t you fix the leak in February?
Real solution? Fire the lazy managers. Pay the cleaners a living wage. Stop pretending tech fixes human failure.
This post reads like a PowerPoint from a consultant who’s never set foot in a factory.
Tejas Bubane
LMAO. You wrote a novel about swabs. Who cares? The real problem? Factories in India and Vietnam are making the same stuff with zero monitoring and selling it on Amazon for $3. Meanwhile, you’re spending $20K/year to test floors. Your product costs 10x. Good luck competing.
Also, ATP? That’s just a fancy way of saying ‘it’s not sticky.’ It doesn’t mean it’s safe. But hey, you can show the auditor a green light. That’s all that matters, right?
This isn’t safety. It’s a tax on small businesses so big players can charge more. Wake up.
Suzanne Johnston
I appreciate the depth here, but I wonder if we’re missing the human layer. In my time working in a UK pharma plant, we had a young technician who started crying because she thought she’d contaminated a batch. She’d swabbed a surface wrong. We didn’t punish her. We sat down, retrained her, and made her part of the training team.
That’s the real magic-not the AI, not the ATP, not the zones. It’s the culture. When people feel safe to admit mistakes, contamination drops. When they’re scared of blame, they hide results.
Maybe the most important ‘monitoring’ tool we have is trust.
Haley P Law
Ok but like… why is everyone so serious?? 😅 I mean, I get it-safety matters. But also… it’s just a lotion tube. 🤷♀️
My cousin works in a plant and they just use bleach and pray. It’s fine. Like, really. People don’t die from a little mold. It’s not like they’re injecting it.
Also, AI? 😭 I can barely get my phone to autocorrect. How’s it gonna predict mold??
Can we just… chill? 😌
Chris Marel
I come from a small town in Nigeria where factories don’t have ATP meters or AI. But they have community. Every morning, the workers gather before shift, check each other’s gloves, remind each other to wash hands. No forms. No logs. Just eyes and respect.
When something goes wrong, they fix it together-not because a rule says so, but because they know the person who eats that food might be their neighbor.
We don’t need more tech. We need more humanity.
Guylaine Lapointe
It’s embarrassing that this is even a conversation. We’re talking about *microbes* in *lotion tubes* like it’s a national security threat. The real contamination is in our heads-the fear that someone, somewhere, might get sick from a product that’s been tested, monitored, certified, and labeled with 17 different compliance stamps.
You know what actually kills people? Poor diet. Lack of clean water. Not enough sleep. Not a single mold spore on a conveyor belt.
This post is a monument to overengineering. It’s not safety. It’s anxiety dressed in lab coats.
Stacy here
Thanks for writing this. As the author, I appreciate the passion-even the angry ones. You’re right: tech doesn’t fix culture. People do. We’re rolling out a new training program next month that pairs every QA tech with a floor cleaner for a week. No reports. Just walk, talk, watch.
Maybe that’s the real Zone 0.