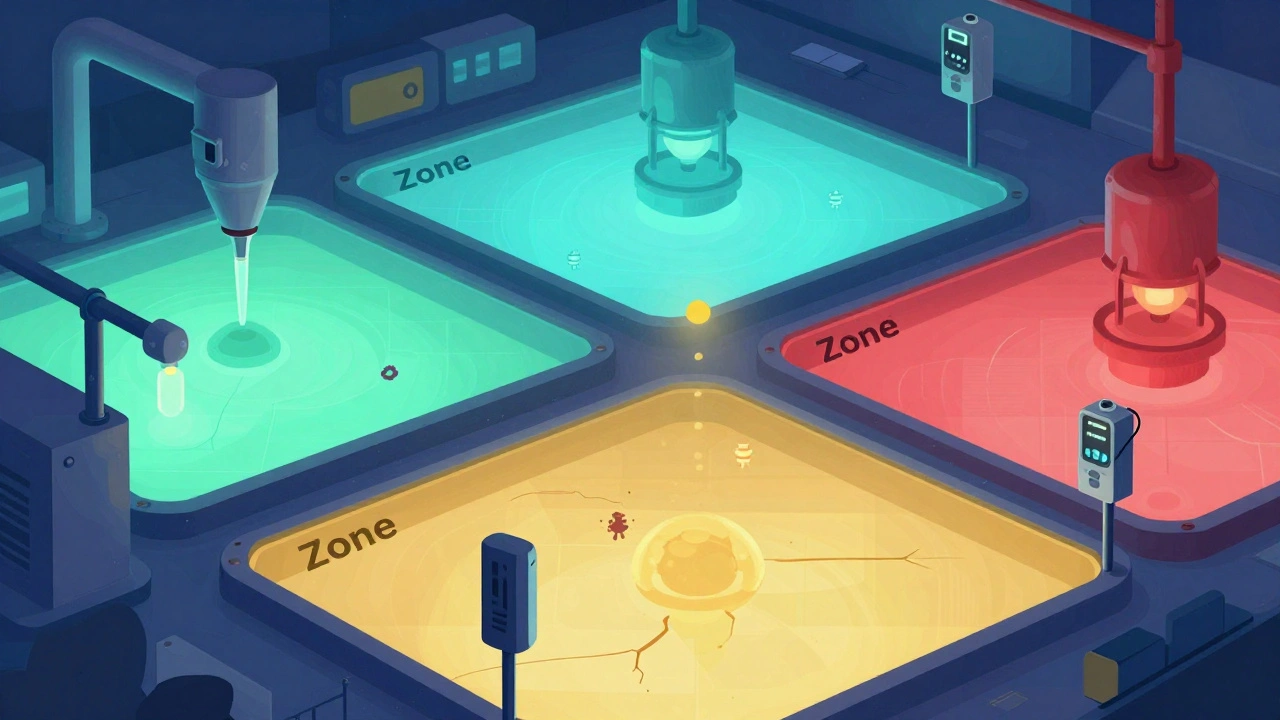
Environmental monitoring is a critical process in manufacturing to detect contamination in air, surfaces, and water before it affects products. Learn how zone-based sampling, industry standards, and new technologies help prevent recalls and ensure safety.
Contamination Testing: How Medications Are Checked for Harmful Impurities
When you take a pill, you expect it to do what it’s supposed to—without hidden dangers. That’s where contamination testing, the process of checking pharmaceutical products for harmful substances like toxins, metals, or leftover chemicals. It’s not just a formality—it’s the last line of defense between you and a dangerous product. This isn’t about dirt or dust. It’s about invisible threats: residual solvents from manufacturing, heavy metals from equipment, microbial growth, or even cross-contamination from other drugs made on the same line. Without strict testing, these could slip through and cause real harm—ranging from mild nausea to organ damage.
drug purity, how clean a medication is at the molecular level is measured against global standards set by the FDA, EMA, and WHO. Labs use high-precision tools like HPLC and mass spectrometry to spot even tiny traces of impurities—sometimes as low as 0.1%. For generics, this is especially critical. A pill might look identical to the brand name, but if the manufacturing process isn’t tightly controlled, impurities can sneak in. That’s why the FDA requires every generic drug to pass the same purity tests as the original. And when a batch fails? It’s destroyed. No exceptions.
pharmaceutical safety, the broader system that keeps medicines safe from factory to pharmacy relies on contamination testing at every stage: raw ingredients, during production, and even after packaging. It’s why your medicine bottle has a lot number—and why recalls happen. Think of it like food safety, but with far less room for error. A single contaminated batch can affect thousands. That’s why companies don’t just test once—they test repeatedly, across multiple samples, using validated methods that can’t be faked.
Contamination isn’t always accidental. Sometimes it’s intentional—like fake pills laced with fentanyl or cheap generics made in unregulated labs. That’s why reporting suspicious meds matters. If your pill looks wrong, tastes odd, or makes you sick, it might not be the drug you think it is. That’s where impurity testing, the specific analysis of unwanted chemical byproducts in medications becomes a public health tool. It’s how regulators catch counterfeiters and how pharmacies avoid stocking dangerous products.
And it’s not just about pills. Injectable drugs, creams, even vitamins go through the same scrutiny. A contaminated nasal spray can cause blindness. A tainted supplement might damage your liver. That’s why contamination testing isn’t just a lab procedure—it’s a daily shield protecting millions. You don’t see it. But every time you take a medicine without getting sick from it, that’s contamination testing at work.
Below, you’ll find real-world stories and breakdowns of how contamination risks show up in everyday meds—from generic pills to emergency kits, from counterfeit drugs to safety recalls. These aren’t theoretical concerns. They’re the reasons why testing exists, why regulations tighten, and why you should always know where your meds come from.
Environmental monitoring in manufacturing prevents contamination by testing air, surfaces, and water for microbes, chemicals, and particles. Learn how zone systems, sampling methods, and regulations ensure product safety in pharma, food, and cosmetics.