How to Check FDA Drug Shortage Database for Medication Availability

Why checking the FDA drug shortage database matters
If you’re a patient, caregiver, or healthcare provider, running out of a prescribed medication can be more than inconvenient-it can be dangerous. In 2024, over 290 drugs were in short supply across the U.S., with many of them being essential medicines like insulin, antibiotics, and IV fluids. The FDA drug shortage database is the only official federal source that tracks which medications are running low, why, and when they might become available again. Unlike pharmacy websites or news alerts, this database gives you verified, up-to-date information straight from the agency responsible for drug safety.
Manufacturers are legally required to report shortages to the FDA under the Food and Drug Administration Safety and Innovation Act (FDASIA). That means if a drug is listed here, it’s not a rumor-it’s confirmed. The database doesn’t just tell you a drug is unavailable; it tells you which specific brand or generic version is affected, who makes it, and whether the shortage is due to production problems, raw material delays, or quality control failures.
How to access the FDA drug shortage database
You don’t need special access or a login to use the FDA drug shortage database. It’s free and open to everyone. Here are the three main ways to check it:
- Web browser: Go to www.accessdata.fda.gov/scripts/drugshortages/default.cfm. This is the most detailed version, with filters, NDC numbers, and full manufacturer details.
- Mobile app: Download the free "FDA Drug Shortages" app from the Apple App Store or Google Play. It’s designed for quick checks on the go and sends push notifications when a drug you’re tracking goes into shortage.
- Data.gov: For developers or institutions, the FDA provides a downloadable dataset updated weekly. This isn’t meant for individual patients but can be used by hospitals or pharmacies to build internal alerts.
The app is especially useful if you take a medication that’s been short before. You can search for your drug by name and turn on alerts so you’re notified immediately if there’s a change in availability. No email signup is required for basic use.
How to search for a specific drug
Searching is simple, but there’s a key detail most people miss: you need to search by the generic name, not the brand name. For example, if you take "Lipitor," you must search for "atorvastatin." The FDA database doesn’t list brand names in its main results.
Here’s how to do it right:
- Open the website or app.
- In the search bar, type the generic name of the drug (e.g., "metformin," "levothyroxine," "furosemide").
- Click "Search."
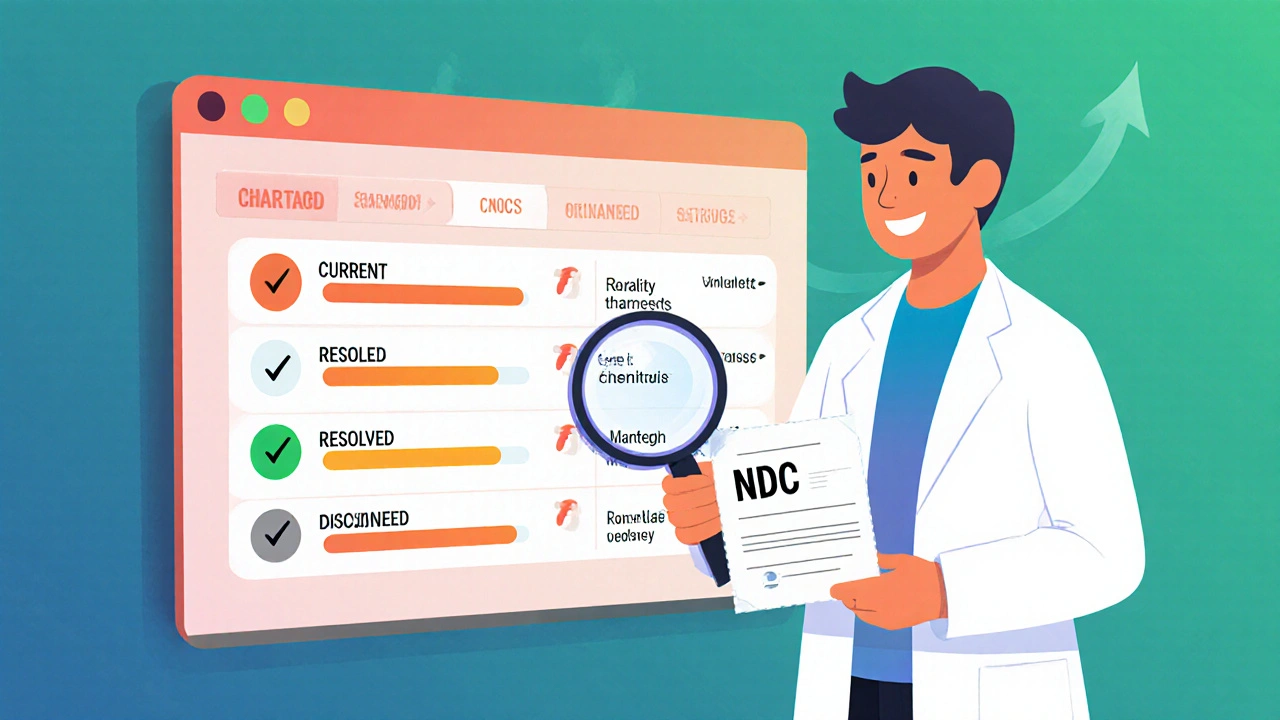
If the drug is in shortage, you’ll see a list of all affected versions. Each entry shows:
- Generic name and active ingredient
- Manufacturer name
- National Drug Code (NDC) number-this is the unique identifier for the exact product you’re prescribed
- Reason for shortage (e.g., "manufacturing delays," "quality issues")
- Current status: "Current," "Resolved," or "Discontinued"
- Estimated resolution date (if available)
Pay close attention to the NDC number. Two versions of the same drug can have different shortage statuses. For example, one manufacturer’s 500mg tablet might be in shortage, while another’s 250mg capsule is fine. If your prescription says "NDC 0002-8745-01," look for that exact number in the results.
Understanding the shortage status and reasons
"Current" doesn’t always mean the drug is completely gone. It means demand is higher than supply. You might still get it at some pharmacies, but with delays or limits. "Resolved" means the FDA believes supply now meets demand-but that doesn’t always mean shelves are fully stocked. Some pharmacies may still be catching up.
The "reason for shortage" field is critical. Over 68% of shortages are caused by manufacturing or quality issues. That means if one version of a drug is affected by a factory shutdown, other versions made by the same company might be at risk too. If the reason is "raw material shortage," it could affect multiple drugs made with the same ingredient.
Some entries include "extended use dates." This means the FDA has approved using the drug past its printed expiration date because of the shortage. These are typically for sterile injectables and critical medications. If you see this note, your pharmacist may have access to extended-dated product. Always ask.
What the FDA database doesn’t tell you
The FDA database is authoritative, but it’s not perfect. It’s reactive, not predictive. A drug might already be unavailable at your pharmacy before it shows up on the site. On average, there’s a 7-10 day lag between when a shortage starts and when the FDA updates its listing.
It also doesn’t tell you what alternatives to use. For example, if your insulin is in short supply, the FDA won’t recommend switching to another brand. That’s where the American Society of Health-System Pharmacists (ASHP) website comes in. Many providers check both: FDA to confirm the shortage, ASHP to find clinical alternatives.
Another gap: the database doesn’t track regional shortages. A drug might be available in Boston but not in rural Texas. The FDA only lists nationwide shortages. If you’re in a remote area and can’t find a drug, it might still be in stock locally-call nearby pharmacies before assuming it’s unavailable.
How to use the database in real life
Here’s a real workflow for patients and caregivers:
- Check the FDA database every time you refill a prescription that’s had shortages in the past.
- Write down the NDC number from your prescription label.
- Search that NDC in the database. If it’s listed, call your pharmacy before going in.
- If the drug is short, ask your pharmacist: "Is there another manufacturer’s version available?" or "Can you get it shipped from another location?"
- If you can’t get your drug, contact your doctor. They can help you switch to an alternative-or request an emergency supply if one exists.
For healthcare providers, the process is similar but includes one extra step: check the "reason for shortage." If it’s a manufacturing issue with a specific company, avoid prescribing that brand even if another version is available. If it’s a raw material issue, multiple drugs might be affected. That helps you plan ahead.
What to do if your drug isn’t listed
If you can’t find your drug on the FDA database but your pharmacy says it’s out of stock, don’t assume it’s not a shortage. The FDA database only includes drugs that meet its national shortage threshold. Some local or temporary issues aren’t reported.
Here’s what to do:
- Call your pharmacy and ask if they’ve received a notice from the manufacturer.
- Check ASHP’s drug shortages page (ashp.org/drug-shortages) for a broader list.
- If you suspect a shortage that isn’t listed, report it. You can email [email protected] with the drug name, manufacturer, NDC, and how long it’s been unavailable.
The FDA encourages reports from patients and providers. Every report helps them catch shortages faster.

Stay updated without checking every day
You don’t have to log in daily. The FDA sends free email updates every Tuesday and Friday with new and resolved shortages. Subscribe at the bottom of the FDA Drug Shortages page.
The mobile app also lets you set up alerts for specific drugs. Once you add a drug to your watchlist, you’ll get a push notification if its status changes. This is the easiest way to stay informed without effort.
What’s next for the FDA drug shortage database
The FDA is working on improvements. By late 2025, they plan to integrate real-time data from wholesale distributors to catch shortages earlier. They’re also testing AI tools that could predict shortages before they happen-using data like production delays, raw material shipments, and historical trends.
For now, the database remains your best tool for accurate, official information. It’s not flawless, but it’s the most reliable source we have. And for a system that handles over 5,000 shortage reports a year, it’s doing a lot with limited resources.
Frequently Asked Questions
Is the FDA drug shortage database free to use?
Yes, the FDA drug shortage database is completely free. There are no subscriptions, fees, or registrations required to search or receive alerts. The mobile app is also free on both iOS and Android.
Can I trust the FDA drug shortage database over my pharmacy’s website?
Yes. Pharmacies often rely on supplier updates, which can be delayed or incomplete. The FDA database is the official federal source with mandatory manufacturer reporting. If a drug is listed as in shortage by the FDA, it’s confirmed nationwide. Always use the FDA database as your primary source, then check with your pharmacy for local availability.
Why does the FDA database list generic names instead of brand names?
The FDA tracks drugs by their active ingredient, not brand names, because multiple companies make the same generic drug. A shortage might affect only one manufacturer’s version, not all. By using generic names, the database helps you identify which specific product is affected and whether other versions are still available.
What if the FDA database says a shortage is resolved, but I still can’t get my medication?
"Resolved" means the FDA believes supply meets national demand, but distribution takes time. Pharmacies may still be restocking. Call your pharmacy to confirm availability. Some may have limited stock or require special ordering. Don’t assume the drug is fully back in stock just because the status changed.
How often is the FDA drug shortage database updated?
The database is updated daily. New shortages, status changes, and resolved items are added every business day. The mobile app syncs with this live data, so you always see the most current information. The downloadable dataset on data.gov updates weekly.
Can I report a drug shortage to the FDA?
Yes. If you’re a patient, pharmacist, or provider and notice a drug is unavailable that isn’t listed on the FDA website, you can report it by emailing [email protected]. Include the drug name, manufacturer, NDC number, and how long it’s been out of stock. Your report helps the FDA identify shortages faster.
Next steps if you’re affected by a shortage
If you’re currently dealing with a medication shortage:
- Don’t stop taking your medication without talking to your doctor.
- Call your pharmacy first-ask if they have another manufacturer’s version.
- Check the FDA database for the exact NDC on your prescription.
- Use the FDA app to set up alerts for future changes.
- If no alternatives are available, contact your prescriber to discuss switching to another drug or getting a temporary supply.
Drug shortages are stressful, but you’re not alone. The FDA database gives you the facts. Use it to make informed decisions-and don’t wait until the last minute to check.


saurabh lamba
lol why do we even need this? 🤡 My pharmacy just tells me what's available and I take it. If it's gone, I ask for something else. Why overcomplicate life with databases and NDC numbers? I'm not a pharmacist.
Kiran Mandavkar
Ah yes, the sacred FDA database-the only temple where truth is encoded in bureaucratic Latin and NDCs. How quaint. Meanwhile, in the real world, people are dying because the system is designed to fail slowly, not to save lives. You think a website fixes capitalism's broken supply chains? Wake up. This is performative transparency with a side of regulatory theater.
Eric Healy
you search by generic name not brand name dumbass lol i cant believe people dont know this. i been using this since 2020. also the app is fire. set alerts for metformin and levothyroxine. saved my ass twice. dont be lazy check it before u drive 20 mins to pharmacy only to get told 'sorry out'
Joseph Townsend
I mean... this is either a miracle or a nightmare. 🎭 One minute you're calmly refilling your insulin, the next you're scrolling through a federal database like you're in a Cold War spy thriller. NDC numbers? Extended use dates? Manufacturers? I didn’t sign up for this. I just wanted my damn pills. But... honestly? This is the most important thing I’ve learned this year. If you’re on meds, this isn’t optional-it’s survival mode. I’m crying. I’m not okay.
Bill Machi
The FDA is doing its job while the rest of the world collapses. This isn’t some ‘convenient tool’-it’s a lifeline. You want to know why America’s healthcare system is broken? Because people ignore this. Because they’d rather complain on Twitter than check a website. If you can’t be bothered to look up your own medication’s status, don’t blame the system when you’re left without insulin. This isn’t politics. This is basic responsibility.
Elia DOnald Maluleke
In the grand tapestry of human suffering, the scarcity of life-sustaining pharmaceuticals is but one thread-yet it is woven with such cruel precision. The FDA database, though a mere digital ledger, becomes in this context a cathedral of hope. One must not merely consult it, but reverence it. For in the NDC code lies the dignity of a human being who requires not convenience, but continuity. Let us not treat this as a chore, but as a solemn duty to our fellow travelers on this fragile earth.
satya pradeep
bro this is gold. i use this daily. just last week my mom's furosemide was out and i checked the database-found another manufacturer with same NDC. called pharmacy, they had it in 2 days. also-always check ASHP too. FDA lags. and yeah, if you're in rural india or africa, same rules apply. if your med's not listed but pharmacy says out? email fda. they actually reply. i did it. they added it next day. you're not just helping yourself-you're helping the system work.
Prem Hungry
To every person reading this: You are not alone. This system is broken, but you are not powerless. Check the database. Set the alerts. Call your pharmacist. Ask for alternatives. Write to the FDA. Your voice matters. Your life matters. You’ve already taken the hardest step-you care. Now go protect it. You’ve got this.