Warfarin and Generic Switching: What You Need to Know About INR Monitoring and Safety

Switching from brand-name Coumadin to a generic version of warfarin might seem like a simple cost-saving move - and for most people, it is. But with warfarin, even small changes can carry big risks. This isn’t just about saving money at the pharmacy. It’s about keeping your INR stable, avoiding dangerous bleeds, and staying out of the hospital. If you’re on warfarin or caring for someone who is, understanding what happens when generics are swapped matters - not just for your wallet, but for your life.
Why Warfarin Is Different
Warfarin isn’t like most pills. Most medications have a wide safety margin - you can take a little more or less and nothing dramatic happens. Warfarin doesn’t work that way. It has a narrow therapeutic index, meaning the difference between an effective dose and a dangerous one is tiny. Your target INR (International Normalized Ratio) is usually between 2.0 and 3.0. Go below that, and you risk a clot. Go above, and you risk bleeding - sometimes severely, even fatally.This is why regular INR checks are non-negotiable. For someone just starting warfarin, doctors often check INR every few days until it stabilizes. Once stable, you might only need testing every four to six weeks. But if you switch from one generic warfarin to another - even if both are labeled "therapeutically equivalent" - your INR can jump or drop unexpectedly. That’s not a myth. It’s backed by real data from clinics across the U.S.
Generic Warfarin Isn’t All the Same
The FDA says all approved generic warfarin products are equivalent to Coumadin. That’s true on paper. But bioequivalence standards for generics require only that the active ingredient falls within 80-125% of the brand’s absorption levels. For most drugs, that’s fine. For warfarin, it’s not enough.There are currently 12 different generic warfarin sodium products approved in the U.S., made by eight different manufacturers. Each uses slightly different inactive ingredients - fillers, binders, coatings - that can affect how quickly or completely the drug is absorbed. That’s why some patients report changes in INR after switching from Teva to Mylan, or from Sandoz to a smaller supplier. One study tracking 3,000 nursing home residents found that nearly one in six had an adverse event related to warfarin, and many were preventable with better monitoring during switches.
It’s not that generics are unsafe. It’s that they’re not perfectly interchangeable. Think of it like switching from one brand of gasoline to another. Both meet the same standards, but your car might run better on one. With warfarin, your body is the car.
What Happens When You Switch?
Most patients - about 80% - transition smoothly from brand to generic or between generics. But 15-20% see their INR shift enough to need a dose change within the first month. That’s why guidelines from the Cleveland Clinic and American Family Physician agree: when switching warfarin formulations, monitor more closely.Here’s what that looks like in practice:
- Check INR within 3-5 days after the switch.
- Check again in 5-7 days.
- If stable, check weekly for the next two weeks.
- Only return to your usual 4-6 week schedule once two consecutive INRs are in range.
Some clinics even do daily INR tests for the first week after a switch - especially if the patient is elderly, has kidney issues, or is on multiple interacting drugs. This isn’t overkill. It’s smart.
Don’t assume your pharmacist’s note saying "substituted for Coumadin" means you’re safe. The label doesn’t say which manufacturer’s version you got. Ask. Write it down. Tell your doctor. If your INR starts drifting without explanation, check the bottle. Was the manufacturer changed?
What Else Can Throw Off Your INR?
A switch in generic brand isn’t the only thing that can mess with your INR. Many everyday things can too:- Diet changes - Eating more leafy greens (kale, spinach, broccoli) increases vitamin K, which lowers INR. A sudden drop in these foods can spike INR.
- Antibiotics - Even a short course of amoxicillin or ciprofloxacin can raise INR by 20-50% within days.
- Herbal supplements - Garlic, ginkgo, St. John’s wort - all can interfere.
- Alcohol - Heavy drinking raises bleeding risk; stopping suddenly can raise INR.
- Medication changes - Adding or stopping blood pressure meds, thyroid pills, or even ibuprofen can shift INR.
That’s why doctors ask so many questions at every visit. It’s not just routine. It’s survival.
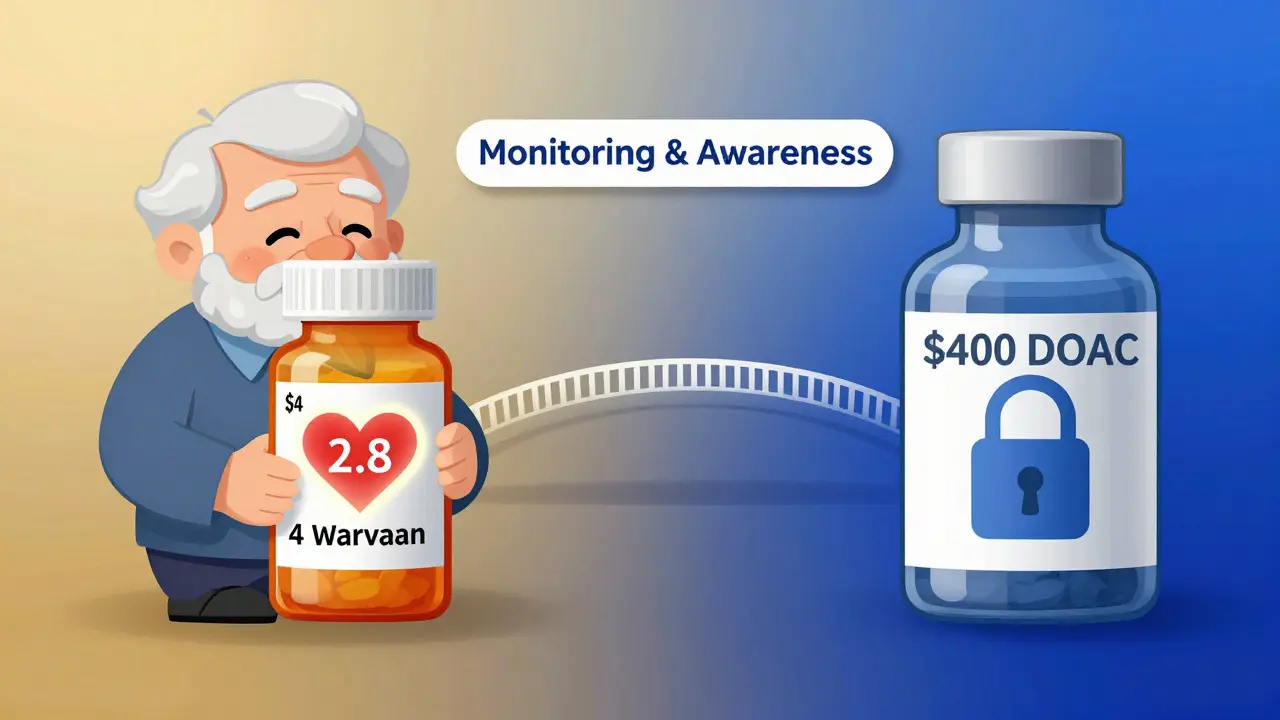
Warfarin vs. DOACs: The Real Trade-Off
Newer anticoagulants - apixaban, rivaroxaban, dabigatran - are often called "DOACs." They don’t need regular INR tests. That’s a huge advantage. But they’re expensive. A month’s supply of a DOAC costs $300-$500. Generic warfarin? $4-$10.For people on Medicare, Medicaid, or without insurance, warfarin is often the only realistic option. It’s also still the only choice for certain conditions: mechanical heart valves (especially mitral), antiphospholipid syndrome, or severe kidney failure. DOACs don’t work well for these cases.
So the real question isn’t "warfarin or DOAC?" It’s "which one works for my life?" If you’re young, active, and have good insurance, a DOAC might be easier. If you’re older, on a fixed income, or have a mechanical valve, warfarin is still the standard - and switching generics is part of managing it.
What Should You Do?
Here’s your practical checklist:- Know your INR target - Is it 2.0-3.0? 2.5-3.5? Ask your doctor to write it down.
- Track your doses - Use a pill organizer. Write down each dose and time.
- Ask your pharmacist - "Which company makes this warfarin?" Write it on your calendar.
- Get tested after any switch - Don’t wait for your next scheduled test. Call for an early INR.
- Report changes - If you feel dizzy, bruise easily, or notice blood in urine or stool, get checked immediately.
- Keep a log - Record your INR values, doses, diet changes, and new medications. Bring it to every appointment.
One patient in Manchester told me she switched generics without telling her doctor. Two weeks later, she had a nosebleed that wouldn’t stop. Her INR was 7.8 - more than double the upper limit. She ended up in the ER. A simple call to her pharmacist before switching could have prevented it.
Bottom Line
Generic warfarin is safe - if you treat it with care. Don’t assume all generics are the same. Don’t skip monitoring after a switch. Don’t ignore diet or drug interactions. Warfarin isn’t outdated. It’s essential. And for millions of people, it’s the only affordable way to stay alive.The key isn’t avoiding generics. It’s managing the transition like the high-risk therapy it is. Stay informed. Stay vigilant. And never let cost savings come at the price of safety.
Can I switch between different generic warfarin brands without checking my INR?
No. Even though all generic warfarin products are FDA-approved as equivalent, small differences in inactive ingredients can affect how your body absorbs the drug. Always check your INR within 3-7 days after switching to a new generic brand. Waiting until your next scheduled test could put you at risk for bleeding or clotting.
Why do some people have trouble after switching to generic warfarin?
Some patients are more sensitive to small changes in drug absorption. Factors like age, liver function, genetics (CYP2C9 and VKORC1 variants), and other medications can make one person’s body respond differently to a new generic formulation. While most people adjust fine, about 15-20% need a dose change after a switch - which is why closer monitoring is recommended.
Is it safer to stay on brand-name Coumadin?
There’s no clinical evidence that Coumadin is safer than generic warfarin. Studies involving over 40,000 patients show no significant difference in bleeding or clotting rates between brand and generic when monitored properly. The main reason to stay on Coumadin is consistency - if you’ve been stable on it for years, switching offers no benefit and adds risk. But if cost is an issue, switching to a generic is safe if you follow monitoring guidelines.
How often should I get my INR checked after switching generics?
Check your INR 3-5 days after the switch, then again in 5-7 days. If both results are stable and in range, check weekly for the next two weeks. Only return to your usual schedule (every 4-6 weeks) after two consecutive INRs are within target. If your INR drifts outside range at any point, contact your doctor immediately.
Can I use a home INR monitor after switching generics?
Yes - and it’s often a good idea. Home INR testing gives you more control and faster feedback, especially after a switch. But make sure your device is calibrated correctly and that you’re trained on how to use it. Always report your results to your care team. Home monitoring doesn’t replace professional guidance - it enhances it.
What should I do if my INR suddenly changes with no obvious reason?
First, check your warfarin bottle - did the manufacturer change? Then review your diet, new medications, or alcohol use. If nothing explains the shift, don’t adjust your dose yourself. Call your doctor or anticoagulation clinic. They may want to repeat the test, check for lab error, or investigate drug interactions. Never guess your way back to a safe INR.


Stewart Smith
Man, I switched generics last year and didn’t think twice. Got a nosebleed at 3 a.m. and thought I was dying. Turned out my INR was 6.9. Never again. Always ask the pharmacist which brand it is now. Write it down like your life depends on it - because it does.
Also, side note: if your pharmacist gives you that blank stare when you ask, just walk out. They don’t know either.
Retha Dungga
warfarin 😩 the silent assassin in your pill bottle 🤫
one day you’re fine next day you’re bleeding out like a leaky faucet 💧🩸
genetics? diet? cheap pills? who knows 😭
but i swear if i die from a pill switch i’m haunting my pharmacist 🕯️
Jenny Salmingo
I’ve been on warfarin for 12 years. My mom had a stroke from a clot because she switched generics and didn’t get checked. I always write down the name on the bottle. I even have a little notebook. It’s not fancy, but it keeps me alive. If you’re on this med, just… be careful. It’s worth the extra step.
Aaron Bales
Stop assuming generics are interchangeable. Period.
Check INR 3-5 days after any switch.
Document the manufacturer.
Track diet, antibiotics, alcohol.
That’s it. Do these five things and you’ll be fine.
Don’t overcomplicate it. Just do the basics.
Lawver Stanton
Okay, so let me get this straight - we’re telling people to treat a $5 pill like it’s a nuclear reactor because some pharmacist swapped the filler from cornstarch to lactose? And now we’re supposed to get blood tests every other day like we’re in a sci-fi dystopia? I mean, sure, I get the math - narrow therapeutic window, blah blah - but this feels like the medical-industrial complex turning a simple drug into a full-time job.
And don’t even get me started on the ‘write it down’ advice. I’m supposed to carry a warfarin journal like it’s a sacred text? My grandma didn’t have a notebook and she lived to 92 on warfarin. What’s changed? The price tag? The corporate logos on the bottle?
Also, why are we still using warfarin at all if it’s this fragile? Why not just make a better version? Is it because generics make more money for the system? Or is it because nobody wants to fund a new anticoagulant that doesn’t require 17 blood tests a month?
Just saying - this whole system feels like a scam wrapped in a clinical guideline.
Sara Stinnett
How quaint. You all treat warfarin like some fragile porcelain doll while ignoring the fact that 80% of patients transition without incident. The real problem isn’t the generic - it’s the medical system’s pathological need to infantilize patients. You don’t need a journal. You don’t need daily INRs. You need to take responsibility for your own health instead of outsourcing it to a clinic that charges $200 for a finger prick.
And let’s be honest - if you’re so terrified of a pill switch, why aren’t you on a DOAC? Oh right - because you’re on Medicaid and can’t afford it. So now we’re criminalizing cost-saving measures? Brilliant.
Warfarin isn’t dangerous. Complacency is. Stop treating patients like toddlers with a chemistry set.
linda permata sari
Oh my goodness, I just read this and cried 😭
I’m from Indonesia and my uncle was on warfarin after his heart surgery - he switched generics because it was cheaper, and he didn’t know anything about INR...
He had a brain bleed. He didn’t make it.
I’m so glad someone wrote this. Please, please, please - tell everyone. This is not just American. This is global. 💔
Someone needs to translate this into Bahasa. I’ll do it if someone helps.
He was 68. He loved mangoes and nasi goreng. He didn’t deserve this.
Brandon Boyd
You got this. Seriously. Warfarin is scary, but you’re not alone.
Start small - write the manufacturer on your calendar. Ask the pharmacist one question. Get your INR checked after a switch.
That’s it. You don’t need to be perfect. You just need to be consistent.
And hey - if you’re using a home monitor? That’s a win. Celebrate that. You’re taking control.
One step at a time. You’re doing better than you think.
Branden Temew
So we’ve turned a life-saving drug into a bureaucratic obstacle course because we can’t be bothered to make a better one.
Warfarin is a 70-year-old molecule. We’ve mapped the human genome. We’ve landed rovers on Mars. And yet, we’re still telling people to track their INR like it’s 1954 because the pharmaceutical industry profits more from this chaos than from innovation.
It’s not that generics are dangerous.
It’s that our system is broken.
And the real tragedy? The people who need this drug the most - the elderly, the poor, the uninsured - are the ones forced to navigate this minefield alone.
So yeah. Check your INR. Write down the brand. But also ask - why does this even exist?
Because someone’s making money off your fear.